Biophysics and Photosynthesis Research
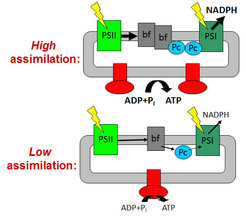
To avoid an over-acidification of the thylakoid lumen and the detrimental production of reactive oxygen species, the photosynthetic ATP and NADPH production is closely adjusted to the ATP and NADPH consumption rates by the Calvin cycle and the subsequent reactions of dark metabolism. High rates of ATP and NADPH production in young leaves with high assimilation capacity are facilitated by high contents of the rate-limiting components cytochrome b6f complex, plastocyanin and ATP synthase. In response to a diminished assimilation capacity, ageing leaves as well as mutants with an impaired Calvin cycle repress photosynthetic electron transport via down-regulation of the cytochrome b6f complex, plastocyanin and ATP synthase. The contents of both photosystems remain largely unaltered, independent of the metabolic demands of the leaf.
Usually, photosynthetic ATP and NADPH production is strictly adjusted to changes in their consumption by the Calvin-Benson cycle and downstream reactions of carbon assimilation, to avoid an over-reduction of the electron transport chain and electron transfer to alternative acceptors such as O2. Otherwise, the whole cellular redox poise might be disturbed, and an increased production of reactive oxygen species could result in the oxidative destruction of the photosynthetic apparatus and initiation of cell death pathways. We mainly focus on adaptive responses occurring on time scales of several hours to several weeks. We aim to obtain an integrated picture of the mechanisms, by which plants adjust photosynthetic ATP and NADPH production to the metabolic demand. For a better understanding especially of slower responses involving altered photosynthetic complex stoichiometry, mechanisms controlling photosynthetic complex accumulation and turnover are determined. With this knowledge, we ultimately will be able to engineer plants with improved photosynthetic performance, stress tolerance, and yield.
Long-term adjustment of photosynthetic electron transport to the metabolic ATP and NADPH demand
Long-term decreases in the metabolic consumption of ATP and NADPH mainly occur during leaf senescence or in response to drought and cold stress. This requires adjustments at the level of photosynthetic complex stoichiometry. Especially the contents of the cytochrome b6f complex, plastocyanin, and the chloroplast ATP synthase strictly correlate with assimilation capacity and, therefore, decrease in response to a reduced metabolic demand for ATP and NADPH. However, the actual metabolic and redox signals, which trigger these adjustments of the photosynthetic apparatus, are largely unknown. Potential candidate signals include reactive oxygen species, photoassimilate accumulation in the leaves (“sugar sensing”), phytohormones and the redox state of the stroma and the photosynthetic electron transport chain itself. To dissect the roles of the different signal classes, we use a wide range of mutants with defined changes in carbon metabolism and sugar sensing, detoxification of reactive oxygen species, and the redox state of the electron transport chain. We determine whether the different signal classes initiate specific adaptive responses of the photosynthetic apparatus. When specific adaptive responses are detected on the level of the light reactions, the underlying mechanisms, from gene expression and translation to photosynthetic complex assembly, maintenance and degradation, are elucidated.
Biogenesis, lifetime and turn-over of photosynthetic complexes
For a deeper understanding of the mechanisms adjusting photosynthetic complex content to the metabolic demand for ATP and NADPH, it is critical to know the lifetimes of the complexes. In case of a highly stable, long-lived complex, its content is mainly regulated on the level of controlled degradation, while in case of a short-lived complex with high protein turnover, its content is mostly regulated on the level of de novo biogenesis. While the biogenesis, lifetime and turnover of PSII have been thoroughly investigated since decades, much less is known about the other photosynthetic complexes. We mainly focus on the cytochrome b6f complex and PSI, using the inducible repression of essential nucleus-encoded structural subunits or of essential auxiliary proteins involved in complex assembly to stop the de novo biogenesis at different time points of leaf development and in different environmental conditions. Then, we follow the loss of the complexes, to determine their lifetimes, and the dependence of their turnover on different developmental states and abiotic stresses. Furthermore, novel auxiliary proteins involved in the biogenesis and turnover of these complexes are identified and functionally characterized.
Photosynthetic adjustments to diurnal changes in the metabolic demand
We also analyze photosynthetic responses to imbalances occurring on an intermediate time scale of several hours. We focus on Crassulacean Acid Metabolism (CAM) plants, which need to cope with strong diurnal changes in leaf assimilation, and also with changes in the metabolic ATP demand relative to that for NADPH. CAM plants do not alter photosynthetic complex accumulation during the diurnal CAM cycle, in line with the long lifetimes of these complexes. Instead, these plants change the sub-compartmentation of the cytochrome b6f complex between grana stacks (where rapid linear electron transport takes place) and unstacked membranes, thereby drastically altering the capacity of linear electron transport within a few hours. The underlying mechanisms are now being elucidated.



