Biophysics and Photosynthesis
The “Biophysics and Photosynthesis” research group of Dr. Schöttler is part of Department 3, Prof. R. Bock, at the MPIMP, in combination with a role as a science infrastructure group.
You can find more information about the group of Mark Aurel Schöttler, their research activities, the group members and publications on the website of Dr. Schöttler's research group.
Service:
1) Gas exchange measurements are performed with three modern gas exchange systems equipped with several measuring heads for gas exchange analyses of leaves of different sizes, and of entire plants. These systems allow a full control of environmental parameters such as temperature, humidity, CO2 and O2 concentration and actinic light intensities. Under these highly controlled conditions, it is possible to determine leaf respiration in darkness, leaf assimilation at different light intensities, and transpiration (evaporation of water from the leaf). Furthermore, derived parameters such as stomatal conductance and ci (the CO2 concentration in the intercellular space), and photorespiration can be determined.
In combination with chlorophyll-a fluorescence measurements (see below), also the role of other alternative electron sinks can be assessed.
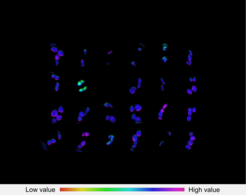
Treatment of Arabidopsis seedlings with a chemical library leads to the identification of one chemical, which represses photosynthetic electron transport, detectable by Chlorophyll-a fluorescence measurements.
2) Chlorophyll-a fluorescence measurements are mainly performed with Imaging-PAMs, which allow us to determine the spatial distribution of photosynthetic parameters such as linear electron flux over the leaf area. Technical options include high resolution images of small leaf areas, or overviews of photosynthesis in whole leaves or even entire plants. In this way, heterogeneous behavior of leaf photosynthesis due to developmental gradients in the leaf, different biotic and abiotic stresses, or due to the local or systemic application of chemical effectors can be resolved.
By coupling the Imaging-PAMs to gas exchange systems (see above), fluorescence imaging measurements can be performed under full control of environmental parameters such as temperature, humidity, CO2- and O2-concentration. Additionally, leaf absorptance measurements for data normalization are performed as part of the service measurements.
3) We also offer 3D imaging measurements on small plants, which can be used to follow plant development and determine changes in plant size, leaf number and leaf area, and the position and angle of the individual leaves. These data can be integrated with the chlorophyll-a fluorescence imaging measurements, to calculate the effective light intensity on the leaf surface. This allows us to precisely determine photosynthetic electron transport rates independent of the leaf angle relative to the light source.
4) As part of a scientific cooperation, more advanced spectroscopic techniques can be applied: These allow the determination of the relative stoichiometry of the photosynthetic complexes and of the thylakoid membrane energization and the activity of chloroplast ATP synthase in intact leaves. Also the precise absolute quantification of all redox-active components of the photosynthetic apparatus in isolated thylakoid membranes is possible.
