Breakthrough in plant breeding
Grafting and mobile CRISPR for genome editing in plants
A ground-breaking twist to the CRISPR tool – aka “genetic scissors” – is being put to use to edit plant genomes by scientists from the Max Planck Institute of Molecular Plant Physiology, signalling a methodology change. The discovery that was recently published in the prestigious journal Nature Biotechnology could simplify and speed up the development of novel, genetically stable commercial crop varieties by combining grafting with a ‘mobile’ CRISPR tool.
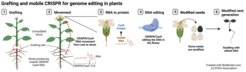
An unmodified shoot is grafted onto roots that contain a mobile CRISPR/Cas9, which allows the genetic scissor to move from the root into the shoot. There it edits the plant DNA but leaves no trace of itself in the next generation of plants. This breakthrough will save time, money and circumvent current limitations in plant breeding and contribute to sustainable food solutions across multiple crops.
Many crops that feed the world are already threatened by heat, drought and plant pests, and these factors are being further exacerbated by a changing climate. To future-proof these essential plants for efficient and effective crop yields under challenging conditions, plant genomes can be edited with high precision using the CRISPR/Cas9 system to introduce beneficial gene functions or to remove unfavourable ones. While CRISPR/Cas9 is an enormous step forward for plant breeding, it remains nonetheless an expensive and laborious solution, making it unfeasible for application in most plants. The recent development made by the team of scientists at the Max Planck Institute of Molecular Plant Physiology in Germany overcomes these limitations.
RNA as CRISPR carrier
Commercial crop plants need to be genetically stable, they cannot contain any genetic sequences from the CRISPR/Cas9 system, and should be transgene free. Normally, this is achieved either through outcrossing over many generations, or via tedious regeneration processes. Both are time and money intensive and are difficult, or even impossible in many crop plants. A team of scientists led by Dr. Friedrich Kragler set out to change this. As part of the EU-funded PLAMORF project and German Ministry of Research funded proof of concept project, they are studying transport sequences that enable the movement of RNAs from roots to shoots. The research group identified the so-called tRNA-like sequences (TLS) that act as signals for the long-distance movement of RNAs within plants. The recent breakthrough came by combining this discovery with the CRISPR/Cas9 genome editing system. When adding such a TLS to the CRISPR/Cas9 sequences, plants produce “mobile” versions of CRISPR/Cas9 RNA. A transgene-free, unmodified shoot is then grafted onto the roots of plants containing the mobile CRISPR/Cas9 RNA, which then moves from the root into the shoot, and eventually on into the flowers that produce the seeds.
“The magic happens in the flowers,” explains Dr. Friedrich Kragler. “The CRISPR/Cas9 RNA moves in and is converted into the corresponding protein, which is the actual ‘genetic scissors’. It edits the plant DNA in the flowers. But the CRISPR/Cas9 system itself is not integrated in the DNA. So, the seeds that then develop from these flowers carry only the desired editing. There is no trace of the CRISPR/Cas9 system in the next generation of plants and it works with a surprisingly high efficiency.”
An editing system for many crop plants
What makes the new system even more exciting is the possibility to combine different species. The scientists showed that ‘editing’ in this way doesn’t only work when root and shoot in grafting are from the same plant species – in this case the model plant Arabidopsis or thale cress. They also grafted shoots of its commercial relative, oilseed rape, onto Arabidopsis roots that produce the mobile CRISPR/Cas9. Encouragingly, the team of Dr. Kragler also found edited oilseed rape plants.
“Our novel gene editing system can be used efficiently for many breeding programmes and crop plants. This includes many agricultural important plant species that are difficult or impossible to modify with existing methods,” he concluded.DF, FK, TL, URS
