The Role of LEA (late embryogenesis abundant) Proteins in Cellular Stress Tolerance
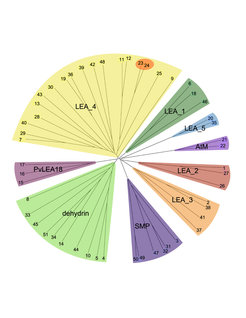
Most of these proteins have no stable secondary structure in solution, i.e. they belong to the large group of intrinsically disordered proteins (IDPs). We performed a comprehensive study of the expression and genomic organizations of the 51 genes encoding LEA proteins in Arabidopsis, with the long term aim of defining their functional significance and mode of action in cellular stress tolerance. It has been found in several plant species that most of the functional damage incurred during desiccation and freezing is the consequence of injury to cellular membranes. We are therefore investigating the ability of recombinant LEA proteins to influence the stability of membranes during freezing or drying. The functional and structural properties of the proteins are investigated using Fourier-transform infrared (FTIR) and circular dichroism (CD) spectroscopy. To study the interactions with membranes, we use artificial lipid vesicles (liposomes) that can be produced with a variety of user-defined lipid compositions. In addition, we use genetic approaches such as RNA interference, overexpression or GFP tagging to study the function and localization of the proteins in planta.
